A range of health and social care research studies apply for approval to go ahead in the UK every year.
These studies use a range of methods and focus on various topics.
On this page you can see a breakdown of some of the different types of studies that have been reviewed in the UK, how many of these studies were reviewed and what percentage of the total number of studies reviewed they account for.
This information gives us valuable insight into the types of research that are underway in the UK. In turn this helps us support REC members with further training on developing areas of interest.
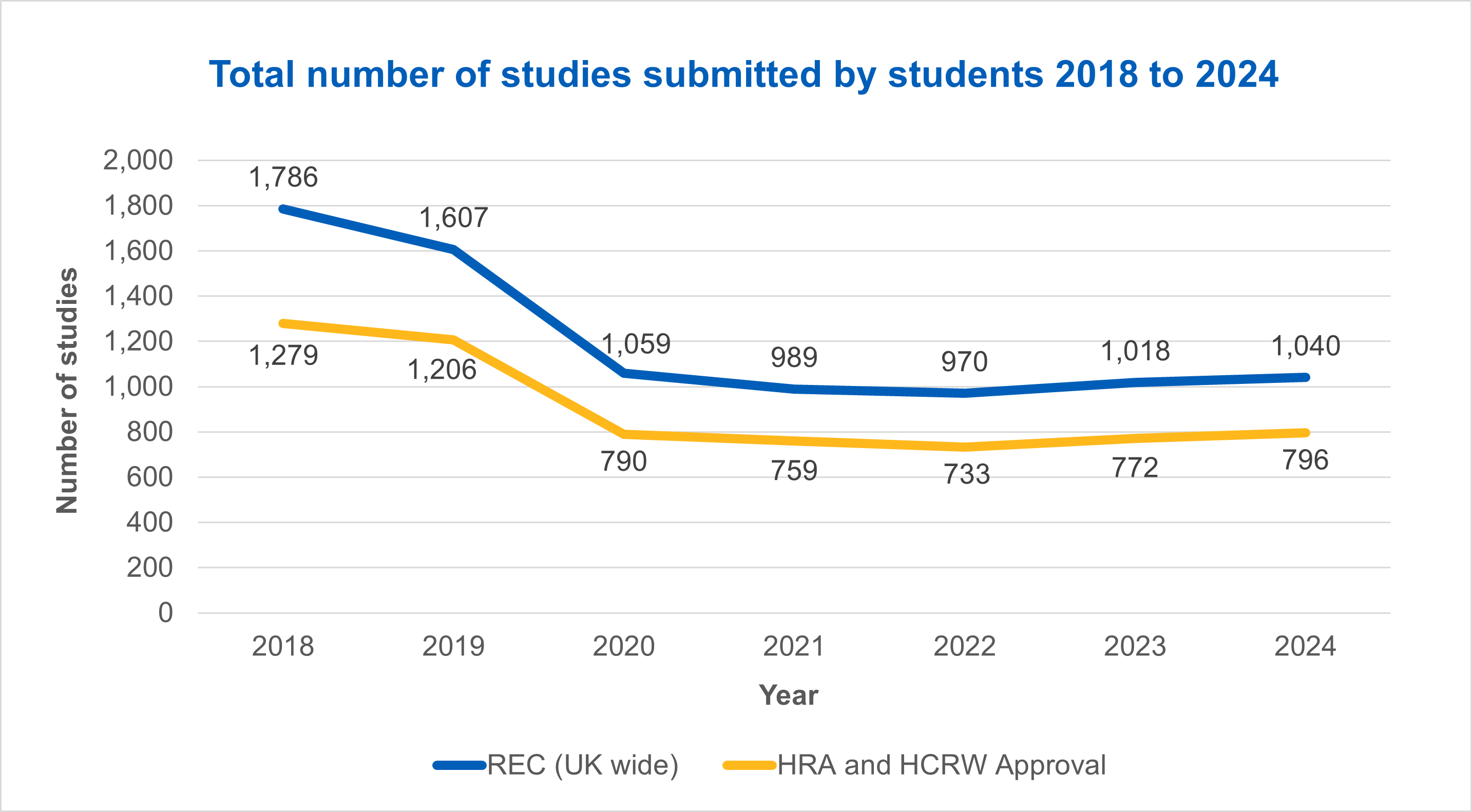
Studies submitted by students
Between 2018 and 2024 we have seen a significant reduction in the number of studies submitted by students receiving REC (UK wide) approval or HRA and HCRW Approval.
From our data we can see a large reduction between 2019 and 2020, falling from 1,607 in 2019 to 1,059 in 2020 for studies receiving REC approval.
For studies receiving HRA and HCRW Approval these numbers dropped from 1,206 in 2019 to 790 in 2020.
These falls represent an 8% drop in the percentage of studies that student research accounts for from the overall number of studies approved by the HRA, falling from 32% in 2019 to 24% in 2023 for REC (UK wide) approved studies and 28% in 2019 to 20% in 2020 for studies receiving HRA and HCRW Approval.
The reduction we have seen is down to changes made to the way student research is carried out in the NHS.
Since 2020 we have seen a return to consistent numbers of studies being approved each year.
Studies involving children
There are specific ethical and legal issues to consider with research involving children (aged under 16) which Research Ethics Committees carefully consider as part of their ethical review.
Over the past seven years we have seen a slight drop in the total number of studies receiving a final opinion from a REC (UK wide) or HRA and HCRW Approval that involve children.
This fall is in line with an overall decrease in the number of studies that we have received and approved between 2018 to 2024.
The most significant fall was seen during the COVID-19 pandemic where numbers fell from 748 (REC UK wide) in 2019 to 662 in 2020, and 577 (HRA and HCRW Approval) in 2019 to 498 in 2020 respectively.
This fall is linked to a focus on COVID-19 related studies during this period where we saw a focus on studies involving older age groups who were disproportionately impacted by the virus.
Despite the fall in the overall number of approved studies, research involving children aged under 16 has consistently represented around 15% (REC UK approval) and 13% (HRA and HCRW Approval) of all studies receiving approval in the UK between 2018 and 2024.

Studies involving adults without capacity to consent
One of the key considerations a Research Ethics Committee will discuss during an ethics review is that of informed consent.
This is particularly important when research participants may lack the capacity to consent or give permission to take part.
Adults who can't consent for themselves who are taking part in a Clinical Trials of Investigational Medicinal Products (CTIMP) are covered by the Clinical Trials Regulations 2004 which are UK wide.
For non-CTIMP studies each nation has its own legislation.
The Mental Capacity Act 2005 provides a comprehensive framework for decision making on behalf of adults (those aged over 16) in England and Wales who are unable to make decisions for themselves.
In Northern Ireland this work is governed by the Mental Capacity Act 2016, and in Scotland provisions are laid out in the Adults with Incapacity Act 2000.
For ease, the data in this area brings together studies, including Clinical Trial of an Investigational Medicinal Products (CTIMPs), reviewed from all four nations.
In 2020 we saw a rise in the number of studies approved in the UK that involved adults without capacity to consent. This is linked to the number of COVID-19 studies carried out in 2020 that involved adults lacking capacity to consent because they were very ill.
As a proportion of the total number of studies reviewed by the HRA, studies involving adults without capacity to consent accounted for around 7% (REC UK wide) and 5% (HRA and HCRW Approval) respectively.
In the years following we saw numbers return to normal levels, as show in the graph below.

Studies involving ionising radiation
Ionising radiation is widely used across the NHS in the diagnosis and treatment of a range of conditions.
The most common examples are x-rays, nuclear scans and radiotherapy.
Under the current regulations Research Ethics Committee (REC) approval is required where participants will be exposed to ionising radiation as part of their involvement in research.
Looking at our data for 2018 to 2024 we have seen small rises and falls in the number studies we have approved that involve ionising radiation that have been approved in the UK.
The average number of studies each year during this period was 613 for those receiving REC (UK wide) approval and 518 for those receiving HRA and HCRW Approval.
During this period, we have also seen a small increase in the percentage of total studies that studies involving ionising radiation account for.
For studies receiving REC (UK wide approval) the percentage has risen from 13% of all studies in 2018 to 15% in 2024. Similarly for studies receiving HRA and HCRW Approval the percentage has risen from 11% in 2018 to 15% in 2024.

How we break down our data
For most applicants to the Health Research Authority, the REC review forms part of the overall HRA and HCRW Approval process. A favourable opinion from a REC is also required for studies which receive support from the Confidentiality Advisory Group.
We have separated our REC review data from our HRA and HCRW Approval data because HRA and HCRW Approval applies only to the NHS in England and Wales. This approval brings together the assessment of governance and legal compliance, undertaken by dedicated HRA staff, with the independent ethical opinion by a REC so that you only need to submit one application.
Some studies submitted for HRA and HCRW Approval do not require REC approval, others do. This is explained more fully in our guidance.
We have broken down our data to reflect this difference, as well as showing timelines for HRA approval of commercial and non-commercially sponsored research.