When we launched Make it Public, our consultation on research transparency in 2019, we knew that we were short of the 100 per cent target for registration, despite this being a condition of Research Ethics Committee approval.
The respondents, those funding and carrying out research as well as those taking part in studies or benefiting from the outcomes had different ideas how to solve the problem, but we heard loud and clear that whatever measures we put in place needed to be simple and easy, and that we needed to make our expectations clear.
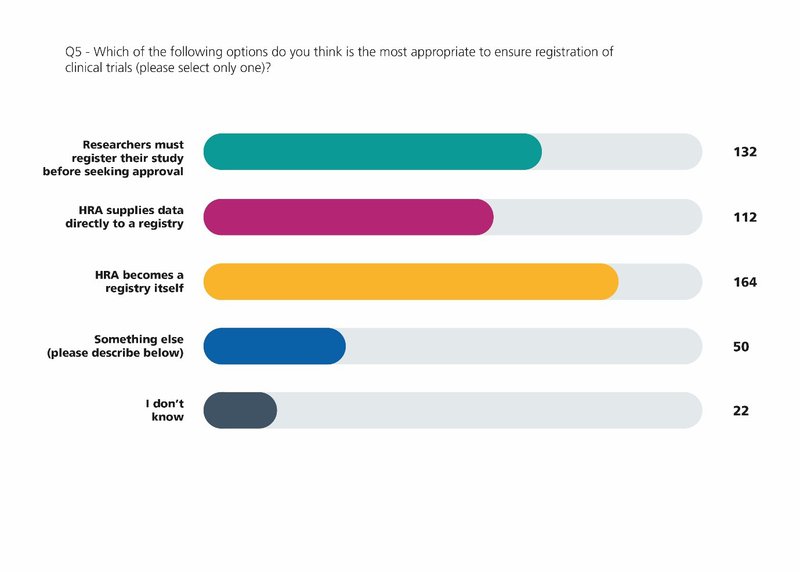
When the Make it Public strategy was published in July 2020 we made a commitment that in the future the HRA will register all clinical trials on behalf of sponsors.
The strategy itself is built upon a foundation of making transparency easy, making transparency the norm, and making information public. The promise of automatic registration is ‘easy’ because we are taking the burden away from the sponsors. Registration becomes the ‘norm’ because we are automating the process – it’s something that happens as a matter of course. And information about all UK clinical trials, active and closed, will be made public in a single location.
There were a number of different ways we could use to make change happen. We asked for help and commissioned an independent agency to assess the options. This led to a partnership with ISRCTN, a World Health Organisation (WHO) recognised UK registry. Working with ISRCTN brings together the resources and expertise of two organisations fully committed to transparency and creates a streamlined process for registration.
The announcement today of the partnership is just one part of a package of measures we are putting in place to improve research transparency, including our new final report, to monitor how researchers are meeting transparency requirements and the annual conference, to make sure that trusted information from health and social care research studies is publicly available for the benefit of all.
In the coming weeks and months, I look forward to talking to people across the research community, and especially those who benefit from the health and social care research approved by the HRA, about the difference being made.

Naho Yamazaki, Head of Policy and Engagement