This report provides a management summary of the activity of the Research Ethics Committees (RECs) in England to enable the HRA Board to undertake its’ function to monitor the performance of the RECs against the requirements of the UK wide Governance Arrangements for Research Ethics Committees (GAfREC).
The following policy and operational changes implemented during the annual reporting period are relevant to the activity and management of RECs over the last 12 months:
Virtual Meetings
In June 2021, members were notified of the proposal to continue to hold REC meetings virtually with RECs holding 10 virtual meetings per year with a reduced number of applications on the agenda (four or five applications per month). Face-to-face development days would be scheduled in order to provide members with the chance to meet in person for learning, team building and to develop relationships.
Biannual regional Chairs meetings have also continued to be held in a virtual format and have been well attended by Chairs and other REC Officers.
Expedited review of COVID-19 studies
The Health Research Authority has continued to provide a fast-track process to review new studies for certain types of COVID-19 research studies, such as vaccine studies. In total, 59 COVID-19 studies have been reviewed through the fast-track process during the annual reporting period.
Ethics Review of COVID-19 human challenge studies
In February 2022, the World Health Organisation (WHO) published a report of a joint Health Research Authority/WHO workshop on the ethics review of COVID-19 human challenge studies. This was held following the establishment of a specialist Research Ethics Committee to review the world’s first COVID-19 human infection challenge study in 2021. The report concluded that the approach adopted by the HRA represented a model of good practice for ethics review of novel, complex, and sensitive study designs.
Combined review
From 1 January 2022, all Clinical Trials of Investigational Medicinal Product (CTIMP) applications have been submitted through the combined review process. RECs which are recognised to review CTIMPs have received training on this process which provides CTIMP applicants and sponsors with a single application route and co-ordinated review, with the REC opinion and MHRA authorisation delivered as a single UK decision.
Fast-track REC
The HRA has continued to provide a fast-track ethics review for global clinical trials and Phase I trials. A Chair of the fast-track REC has been appointed and the Committee which is comprised of a rota of members from a number of recognised RECs meets weekly to offer a rapid ethics review.
Student research
New eligibility criteria were introduced for student research in September 2021. The new criteria specify which types of educational research can be accepted for REC review, and a number of alternatives for undergraduate and master’s studies to gain experience of research have been published.
Membership
The membership of the 64 RECs is set out in figure 1 below. Although GAfREC allows for up to 18 members to be appointed to each REC, 15 members is the operational target. As at 31 March 2022, there were 805 members. Therefore, there was a shortfall of 156 members at the end of the reporting period.
The constitution of RECs is defined in GAfREC and requires a minimum of a third of the membership to be lay. For RECs reviewing CTIMPs, half of the lay members should be unconnected with the conduct of clinical research and not be registered health and social care professionals. In order to ensure sufficient clinical and expert input, the HRA tries to ensure that at least 50% of the members of each REC are expert.
Recruiting experts, particularly clinicians, has continued to be challenging during the year. At the end of March, an advertising campaign was produced to encourage more healthcare professionals to become REC members, however it will be important to continue exploring new and innovative ways of recruiting expert REC members over the next 12 months.
The recruitment of new members is by an open process. Interview panels by video-conference are held on a regular basis and the move to virtual meetings has given greater flexibility to appoint members outside of their geographical location.
At the end of the reporting period the membership of each REC ranged from eight to 15 members. Where meetings were in danger of not being quorate, this was managed by co-opting members from other RECs. Holding meetings virtually has given greater flexibility to allow more members to co-opt to meetings regardless of where the REC is located geographically.
Figure 1: REC Membership

(Note: lay plus members are defined as people who are not, or never have been, a registered health care professional or been involved in the conduct of clinical research; expert members are defined as currently registered health care professionals, individuals with professional qualifications or experience in clinical research or a previously registered doctor or dentist.)
As shown in figure 2 below, a greater number of expert and lay plus members have left the service than we have been able to appoint. This is due to it becoming increasingly difficult to appoint expert members. Work commitments was the main reason cited for members leaving the service with other reasons such as the high workload of being a REC member and missing the social interaction of face-to-face meetings also being factors.
The term of office is five years, which may be renewed, with short-term extensions in exceptional circumstances to maintain the service.
Data on current and predicted vacancies is routinely collected to aid improved succession planning and targeted recruitment.
Figure 2: Number of Members appointed and leaving the service during 2021/22

Download a csv file for graph data from figure 2
Reviews undertaken
Applications reviewed at full REC meetings
The number of applications reviewed at full REC meetings was slightly less than the previous year (2674 in 2021/2022 compared to 2736 in 2020/2021) due to a decrease in non-COVID-19 related research activity and the slower study set-up of existing projects as a result of the pandemic.
The data for some of the CTIMPs included below in tables one to five include applications submitted during the Combined Ways of Working pilot where the clock was not paused whilst applicants prepared their response to requests for further information. This means that the timelines for some of the CTIMP applications included in the figures are not directly comparable to non-CTIMPs where the clock is always paused once the initial outcome is issued.
| Type of study | Number reviewed | Median time to final opinion | Reviewed in less than 60 days |
| CTIMP* (* Timeline for REC in legislation is 60 days) | 794 | 33 days | 89% |
| Non-CTIMP | 1880 | 31 days | 98% |
| Total applications | 2674 | 31 days | 96% |
Applications reviewed by Proportionate Review Sub-Committees
The number of Proportionate Review (PR) applications reviewed in 2021/22 is slightly less than the previous year (751 in 2021/22 compared to 772 in 2020/21). All RECs continue to provide two PR application slots on a monthly basis for 11 months of the year.
|
Number of PR applications reviewed |
Median time to final opinion |
| 751 | 18 days |
Substantial amendments
The number of substantial amendments reviewed by RECs in England has slightly reduced this year (7175 substantial amendments in 2021/22 compared to 7426 in 2020/21).
| Type of study |
Number of substantial amendments reviewed |
Median time to opinion |
Reviewed in less than 35 days |
| CTIMP | 4383 | 21 days | 91% |
| Non-CTIMP | 2792 | 19 days | 94% |
| Total amendments | 7175 | 19 days | 91% |
Modified amendments
Modified amendments can be submitted after an unfavourable opinion of a previous substantial amendment and have a 14 day ethics review timeline.
|
Number of modified amendments reviewed |
Median time to opinion |
| 31 | 13 days |
Section 30 amendments
These are amendments to include adults lacking capacity in the study for the first time and have a 60-day ethics review timeline, as these types of amendments are reviewed at a full Committee meeting in order that the REC can consider whether to approve the study under the Mental Capacity Act.
|
Number of section 30 amendments reviewed |
Median time to final opinion |
| 9 | 27 days |
REC meeting decisions
Individual decision data has been produced for all RECs which has demonstrated a high level of consistency between the types of decisions issued for full and PR applications. RECs which have been identified as outliers have been followed up directly to discuss the reasons behind this.
Figure 3: Applications reviewed at full REC meetings – decision at first review

Download a csv file for graph data from figure 3
- favourable opinion – no outstanding ethical issues and no changes needed
- favourable opinion with conditions – no outstanding ethical issues, minimal specific changes not requiring further review by the REC. Please note, this decision is not able to be issued for combined review CTIMPs and Phase 1 studies.
- provisional opinion – ethical issues to be addressed or further information needed to enable the REC to make a decision.
- unfavourable opinion – significant, unresolved ethical issues. Please note, this cannot be issued for a combined review CTIMP at the time of the first review.
Figure 4: Applications reviewed by Proportionate Review Sub-Committee

Download a csv file for graph data from figure 4.
Fast-track REC
The fast-track REC was tested as a pilot from 31st March 2021 to 30th June 2021 and was then incorporated into business as usual. An evaluation of the fast-track REC to assess the continued provision of this function is currently being undertaken.
The fast-track REC is comprised of a range of members from recognised RECs across the UK who have volunteered to also be involved in the review of fast-track applications and amendments on a rota basis.
Figure 5: fast-track Membership

| Type of submission | Number reviewed |
Median time to final opinion |
| Fast-track Applications | 88 | 14 days |
|
Fast-track Substantial amendments |
175 | 9 days |
Generic Review Committee
This is a small committee of 3 members which was established to provide an ethical opinion on generic recruitment documents for Phase 1 trials. This year has also seen several organisations submitting generic COVID-19 related materials for review by the Committee. As well as new submissions, the Generic Review Committee also receives amendments to previously approved submissions.
| Type of application | Number reviewed |
Median time to final opinion |
| New submissions | 155 | 6 days |
| Amendments | 134 | 2 days |
Accreditation scheme for the audit of RECs
The accreditation scheme audits on the standards that relate directly to RECs. For example, constitution (review of member expert/lay capacity), officer appointments, appointment/terms and conditions, training and attendance, along with final REC meeting products (minutes and decision letters). This scheme runs on a two-year rolling cycle of audits.
No committees failed to achieve accreditation. For those committees that had to complete an action plan before achieving accreditation the most common action related to REC members not having completed their annual training requirement.
Figure 6: Audit outcomes

Appeals
Three appeals against an unfavourable opinion for applications were received; all were allowed resulting in the following outcomes:
- two were issued unfavourable opinions and one was given a further information unfavourable opinion.
Two appeals against an unfavourable opinion for substantial amendments were received; all were allowed resulting in the following outcomes:
- one was resubmitted as a modified amendment and given an unfavourable opinion and one was withdrawn by the applicant.
Breaches
A 'serious breach' is defined as a breach of the protocol or of the conditions or principles of Good Clinical Practice (or equivalent standards for conduct of non-CTIMPs) which is likely to affect to a significant degree the safety or physical or mental integrity of the trial subjects, or the scientific value of the research.
It is a condition of the REC favourable opinion for the sponsor to notify the REC and relevant regulatory bodies of a serious breach in any study within seven days of the matter coming to their attention.
When reviewing breaches, the REC will consider what corrective and preventative actions have been taken and will consider the ethical issues, such as the information provided to participants and any impact on consent previously given.
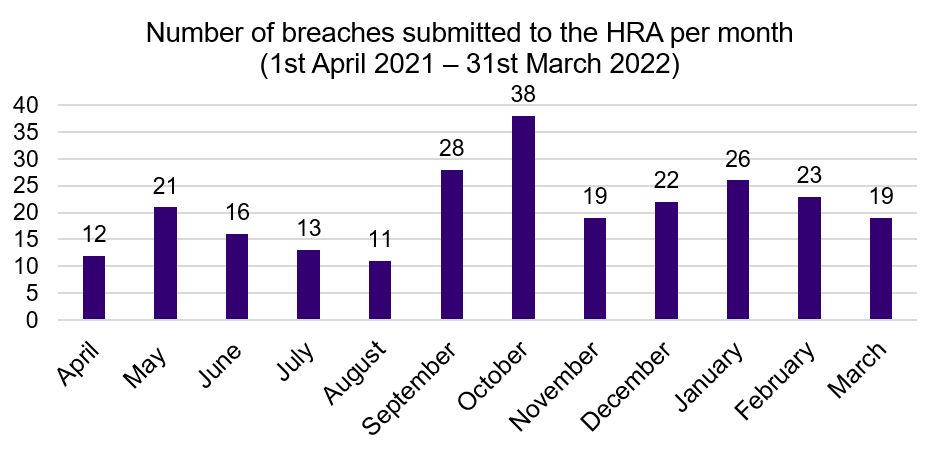
Download a csv file for graph data from figure 7

Download a csv file for graph data from figure 8
Conclusion
RECs have continued to adapt well to the challenges of working in different ways since the onset of the COVID-19 pandemic in March 2020. Virtual REC meetings have many advantages for researchers and REC members, however we are aware that opportunities to meet in person are also very important to members and annual Member Development Days are being held from April 2022 onwards. These will provide opportunities for social interaction between members, as well as valuable opportunities for shared learning to improve knowledge and consistency.
A number of REC members have engaged with the Ethics Review Programme ‘Think Ethics’ by participating in workshops and webinars. We expect to make further improvements to the ethics review process over the next 12 months as a result of the recommendations from this programme of work and we will be working closely with members to achieve this.
Members have continued to deliver an excellent service in challenging circumstances, and we are extremely thankful for the significant and valuable contributions which REC members provide in terms of making the UK an excellent place to conduct health and social care research.
Our work in the HRA to attract and recruit new members with an emphasis on increasing diversity and maintaining a balance of expertise will continue over the next 12 months, as we seek to make the most of member’s valuable time, while aiming for a research ethics review service that is more reflective of the population it protects.